The final temperature on increasing the pressure to 7.65 atm is 637 K.
Step-by-step explanation:
The relation between temperature attained by gas molecules at varying pressure is defined by Guy-Lusac's law. It states that at constant volume, the pressure experienced by the gas molecules is directly proportional to the temperature attained by those molecules.
P∝T
Here, the initial pressure P₁ is given as 4 atm at temperature T₁ = 333 K, then the final pressure P₂ = 7.65 atm can be attained at temperature T₂.
The final temperature should be greater than the initial temperature as there is an increase in the pressure.
So,
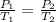
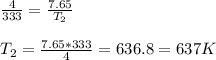
Hence, the final temperature on increasing the pressure to 7.65 atm is 637 K.