Dissolving 0.729 mol NaCl in 2530 mL of water yields a solution with 2.88M concentration.
Step-by-step explanation:
The moles of NaCl given will be dissolved and volume will be made with given data will give molarity.
The units for molarity are moles/litre, so the volume given in ml will be converted to litres by dividing it with 1000.
data given:
number of moles of NaCl = 0.729 moles
volume of the solution = 2530 ml or 2.53 litre
To calculate the concentration of the solution the formula used is:
molarity =
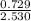
= 0.288 M
The concentration or molarity of the solution OF NaCl is 2.88M