Answer:
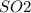 Solutions i.e Sulphur (IV) Oxide solution
Solutions i.e Sulphur (IV) Oxide solution
Step-by-step explanation:
SULFUR (IV) OXIDE is a gas with a toxic smell like burnt matches, and turn moist litmus pink. It can be manufactured as by product of copper extraction.
CONDUCTIVITY refers to measurement of ability of solutions or substance to conduct electricity.
CONDUCTIVITY PROBES ; Is used to measure ectrical conductivity in solutions and can be used to determine the total ion concentration of aqueous solution.
The conductivity probes does not detect electric current with SULPHUR (IV) OXIDE solution because charge cannot move through it when the coductivity probe was used to carry out test on it.