Answer:
The concentration of [Ag⁺] and [PO₄³⁻] is 0.4485M and 5.83 × 10⁻⁶M after precipitation
Step-by-step explanation:
The volume of the mixture formed by mixing 0.100L of AgNO₃ and 0.100L of Na₃PO₄
0.100L + 0.100L = 0.200L
The ionic equation of AgNO₃
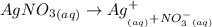
The number of moles of the Ag⁺ in the mixture is calculated by
Number of mole = Molarity × Volume
= 0.310 × 0.100
= 0.031mole
The ionic equation for the Na₃PO₄³⁻
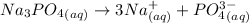
Number of mole = Molarity × Volume
= 1.0 × 0.100
= 0.100 mole
One mole of Ag⁺ react three mole of PO₄³⁻, the number of mole of PO₄³⁻ react with 0.031mole of Ag⁺ is
number of mole PO₄³⁻ =
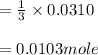
Total number of mole remaining is
0.100 mole - 0.0103 mole
= 0.0897 mole
The concentration of PO₄³⁻ is calculated as shown below
Molarity = number of mole / volume
Molarity = 0.0897 / 0.2
= 0.4485M
The concentration of Ag⁺ is calculated as shown below
![K_p = [Ag^+]^3[PO^3^-_4]](https://img.qammunity.org/2021/formulas/chemistry/high-school/bwtn0cum0vvbk6uppdzqraadlt360dxlo0.png)
![8.89 * 10^-^1^7= [Ag^+]^3(0.4485M)](https://img.qammunity.org/2021/formulas/chemistry/high-school/47znbu9au0elesp1kk5pgy33njpe8r2w3x.png)
![[Ag^+]^3 = (8.89* 10^-^1^7)/(0.4485) \\](https://img.qammunity.org/2021/formulas/chemistry/high-school/tvgz0q0fj4u3x3iwbt2tlpd7vn6nofmcqa.png)
![[Ag^+]^3 = 1.982 * 10^-^1^6](https://img.qammunity.org/2021/formulas/chemistry/high-school/ikm5elpmvc2kop65kuqr5635ga0q5iw7sf.png)
![[Ag^+] = 5.83 * 10^-^6M](https://img.qammunity.org/2021/formulas/chemistry/high-school/k4dobooaascnn8ley1ooa0egz1a3puoub2.png)
The concentration of [Ag⁺] and [PO₄³⁻] is 0.4485M and 5.83 × 10⁻⁶M after precipitation