Answer:
The molarity of this
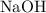 solution is approximately
solution is approximately
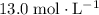 .
.
Step-by-step explanation:
The molarity of a substance gives its quantity (in number of moles) in each liter of the solution.
According to the question, there are
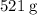 of
of
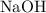 in each liter of this solution. The goal is to find the number of moles of formula units in that
in each liter of this solution. The goal is to find the number of moles of formula units in that
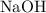 contains three elements: Na, O, and H. Look up a modern periodic table for the relative atomic mass of each element:
contains three elements: Na, O, and H. Look up a modern periodic table for the relative atomic mass of each element:
- Na: 22.990.
- O: 15.999.
- H: 1.008.
Calculate the formula mass of
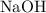 .
.
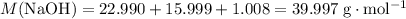 .
.
That's the mass of each mole of
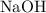 formula units. Calculate the number of moles of formula units in that
formula units. Calculate the number of moles of formula units in that
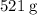 of
of
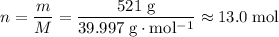 .
.
Apply the equation
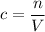 to find the molar concentration
to find the molar concentration
 of this solution. In this equation,
of this solution. In this equation,
 is the number of moles of the solute, and
is the number of moles of the solute, and
 is the volume of the solution.
is the volume of the solution.
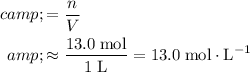 .
.