Answer:
0.00446 grams of hydrogen are produced.
Step-by-step explanation:
Atmospheric pressure at which hydrogen gas was collected = p
p = 742.1 mmHg
Vapor pressure of the hydrogen gas = p' = 22.4 mmHg
Pressure of the hydrogen gas = P
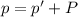
P = p - p' = 742.1 mmhg - 22.4 mmhg = 719.7 mmHg
719.7 mmHg =
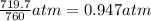
1 atm = 760 mmHg
Volume of the hydrogen gas ,V = 57.48 mL = 0.05748 L
1 mL =0.001 L
Temperature of the hydrogen gas = T = 24°C=24+ 273 K = 297 K
Moles of hydrogen gas = n
 ( Ideal gas equation)
( Ideal gas equation)
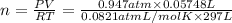
n = 0.00223 mol
Mass of 0.00223 moles of hydrogen gas:
0.00223 mol × 2 g/mol = 0.00446 g
0.00446 grams of hydrogen are produced.