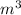Answer:
The volume inside the balloon is = 121
Step-by-step explanation:
Temperature T = - 1 °c = 272 K
Pressure = 0.7 atm = 71 k pa
No. of moles = 3.8
Mass of the gas inside the volume = 3.8 × 4 = 15.2 kg
From ideal gas equation
P V = m R T
Put all the values in above formula we get
71 × V =15.2 × 2.077 × 272
V = 121
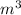
Therefore the volume inside the balloon is = 121