Answer:
The energy absorbed when 6.20 grams of aluminum is heated from 10.0°C to 65.0°C is 306.9 Joules.
Step-by-step explanation:
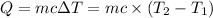
Where:
Q = heat absorbed or heat lost
c = specific heat of substance
m = Mass of the substance
ΔT = change in temperature of the substance
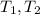 : Initial and final temperature of the substance
: Initial and final temperature of the substance
We have mass of aluminium = m = 6.20 g
Specific heat of aluminium= c = 0.900 J/g°C
Initial and final temperature of the aluminium
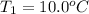
Final temperature of the aluminium =
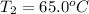
Heat absorbed by the aluminium:
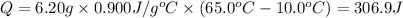
The energy absorbed when 6.20 grams of aluminum is heated from 10.0°C to 65.0°C is 306.9 Joules.