Answer:
A disproportionation redox reaction
Step-by-step explanation:
1. Assign an oxidation number to every atom in the equation
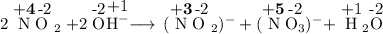
2. Identify the atoms that change their oxidation number
N in NO₂: +4 ⟶ +3 in NO₂⁻
N in NO₂: +4 ⟶ +5 in NO₃⁻
3. Identify the type of change
This is a disproportionation — a reaction in which one substance is simultaneously oxidized and reduced.