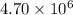Answer : The value of the rate constant for the forward reaction at 700 K is,
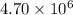
Explanation :
The given chemical equilibrium reaction is:
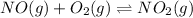
The expression for equilibrium constant is:
![K_c=([NO_2])/([NO][O_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/lu54pd48ktad5pcuacbicm0wnwf4spa0mf.png)
The expression for rate of forward and backward reaction is:
![R_f=K_f[NO][O_2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/mzl1y7nj3n6b4w6mjo31l02lrurzixax94.png)
and,
![R_b=K_b[NO_2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/h63hmrgf62xk9m1dfr6fkaxfqesj4yqdel.png)
As we know that at equilibrium rate of forward reaction is equal to rate of backward reaction.
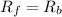
![K_f[NO][O_2]=K_b[NO_2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/mubbotz2vgnbojobq1mythzd6a5bi9cnih.png)
![(K_f)/(K_b)=([NO_2])/([NO][O_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/e91j574fhq5zg6odn6z4g73w442p1gt7a0.png)
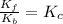
Given:
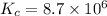
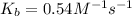
Now put all the given values in the above expression we get:
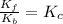
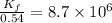
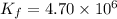
Therefore, the value of the rate constant for the forward reaction at 700 K is,