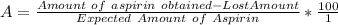Answer:
The weight of aspirin lost is
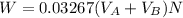
The percent yield is lowered by
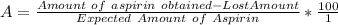
Step-by-step explanation:
From the question we are told that
Since the solubility of aspirin in water is 1 g per 300 mL it implies that after crystallization the solution would contain 1 g for every 300mL of water at 25°
Let assume that the volume of the solution is
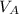
The the aspirin lost after filtration would be
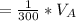
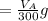
Let assume that you used water of volume
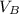 to wash the crystallized aspirin then the lost during washing would be
to wash the crystallized aspirin then the lost during washing would be
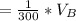
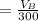
So the total loss is
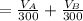
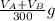
So the weight of aspirin lost denoted by W is
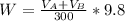
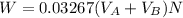
Let denote How much was your percent yield lowered by this loss by A
So