Answer:
The glassware appropriate for dilution preparation is a (250 mL) volumetric flask
Step-by-step explanation:
We know that molarity or concentration of a solution is the number of moles per litres of solution, the molarity and volume of the solution in question can be used to find out how much of the stock solution to be diluted using the formula;
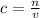
Once the variables are confirmed, a 250 mL solution of 1.50M HCl can be prepared by measuring determined stock solute into a 250mL volumetric flask and the making it upto the 250mL mark. (a measuring cylinder can be used to check accuracy of volume added).