Answer:
The concentration of hydrogen ion in the solution was
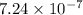 .
.
Step-by-step explanation:
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([NaX])/([HX]))](https://img.qammunity.org/2021/formulas/chemistry/college/kj6uvbf9sz3okr9hr9a5h54v2t99cs8ggj.png)
We are given:
 = Acid dissociation constant of HX =
= Acid dissociation constant of HX =
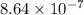
![pK_a=-\log[K_a]=-\log[8.64* 10^(-7)]=6.06](https://img.qammunity.org/2021/formulas/chemistry/college/ckyc5sizdpnm4sk9a85wnx8ls6w26i7fvk.png)
![[salt]=[NaX]=0.803 M](https://img.qammunity.org/2021/formulas/chemistry/college/9iczko1lx68vx29b1etjvz8hnmfx9mqua1.png)
![[acid]=[HX]=0.677 M](https://img.qammunity.org/2021/formulas/chemistry/college/cnpaspcf8jd94sn2xf9mgd9u251v9utq1v.png)
pH = ?
Putting values in above equation, we get:
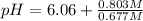
pH = 6.14
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![6.14=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/527yavtu783qcqdfucnmuqr93acva2zgk5.png)
![[H^+]=10^(-6.14)=7.24* 10^(-7)](https://img.qammunity.org/2021/formulas/chemistry/college/uoop7y3u64ax2mctt268cogy9o16il1w18.png)
The concentration of hydrogen ion in the solution was
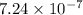 .
.