Answer:
K=1.93
Step-by-step explanation:
Given reactions are:
A +2B ⇄ 2C K1=2.79
2C ⇄ D K2=0.186
D⇄ A+ 2B K=?
If we reverse the reaction then the euillibrium constant also become reciprocal of original value:
3rd reaction is a combination of reciprocal of 1st and 2nd reaction
2C⇄A +2B 1/K1;........................1
D ⇄ 2C 1/K2; ..............................2
On adding 1 and 2
D ⇄A +2B
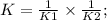
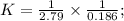
K=1.93