Answer:
The value of Δ
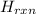 for the reaction = 463
for the reaction = 463
Step-by-step explanation:
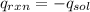
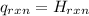
Δ
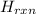 = -
= -
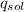 ------ (1)
------ (1)
We know that
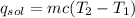
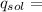 (1)(100) × 4.18 × (32.8 - 25.6)
(1)(100) × 4.18 × (32.8 - 25.6)
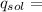 3010 J = 3.01 Kilo Joule
3010 J = 3.01 Kilo Joule
From equation (1)
Δ
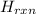 = 3.01 Kilo Joule
= 3.01 Kilo Joule
No. of moles
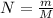
m = 0.158 gm & M = 24.31 gm Mg
No. of moles
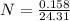
N = 0.0065
Therefore
Δ
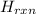 =
=
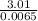
Δ
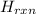 = 463
= 463
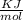
This is the value of Δ
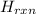 for the reaction.
for the reaction.