Answer:
The value of Ka

It is a weak acid
Step-by-step explanation:
From the question we are told that
The concentration of
![[HClO_2]=0.24M](https://img.qammunity.org/2021/formulas/chemistry/college/363htmkoognv2pcwd1j6subjqu0xhv9lga.png)
The concentration of
![[H^+]=0.051M](https://img.qammunity.org/2021/formulas/chemistry/college/y1ccknnmf0berq1n5wvpxlmfykpgxrx37k.png)
The concentration of
![[ClO_2^-]=0.051M](https://img.qammunity.org/2021/formulas/chemistry/college/zfkz5jh6p3admooxnt1y82b0a7h14y2pd3.png)
Generally the equation for the ionic dissociation of
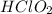 is
is

The equilibrium constant is mathematically represented as
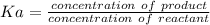
![= ([H^+][ClO_2^-])/([HClO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/m4t7l629vvx6glfa0if8glrkufp8rx2vkd.png)
Substituting values since all value of concentration are at equilibrium
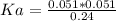

Since the value of is less than 1 it show that in water it dose not completely
disassociated so it an acid that is weak