The given question is incomplete. The complete question is:
Aqueous hydrochloric acid (HCl) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H2O). If 1.60 g of sodium chloride is produced from the reaction of 1.8 g of hydrochloric acid and 1.4 g of sodium hydroxide, calculate the percent yield of sodium chloride. Be sure your answer has the correct number of significant digits in it.
Answer: Thus the percent yield of sodium chloride is 78.0%
Step-by-step explanation:
To calculate the moles :
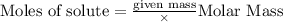
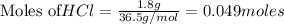
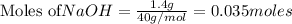

According to stoichiometry :
1 mole of
 require = 1 mole of
require = 1 mole of

Thus 0.035 moles of
 will require=
will require=
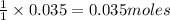 of
of

Thus
 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
 is the excess reagent.
is the excess reagent.
As 1 mole of
 give = 1 mole of
give = 1 mole of
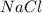
Thus 0.035 moles of
 give =
give =
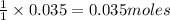 of
of
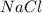
Mass of
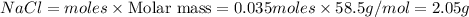
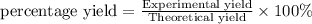
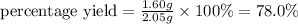
Thus the percent yield of sodium chloride is 78.0%