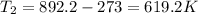Answer:
619.2 K
Step-by-step explanation:
We can solve this problem by using Charle's Law, which states that:
"For a fixed mass of an ideal gas kept at constant pressure, the volume of the gas is directly proportional to its absolute temperature"
Mathematically:
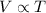
where
V is the volume of the gas
T is the absolute temperature
The equation can be rewritten as follows for a gas that undergoes a transformation at constant pressure:
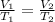
where in this problem:
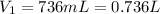 is the initial volume of the gas
is the initial volume of the gas
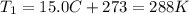 is the initial temperature
is the initial temperature
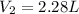 is the final volume of the gas
is the final volume of the gas
Solving for T2, we find the final temperature:
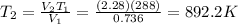
Converting into Celsius degrees,