Answer: The maximum amount of C that can be produced is 2.0 mol.
Step-by-step explanation:
The balanced equation will be as follows.
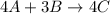
As we are given that,
moles of C = moles of A =
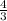 moles of B
moles of B
When we react 2.0 mol A with 3.0 mol B then the limiting reagent is A as specie A has less number of moles. Therefore, the maximum amount of C which can be produced is as follows.
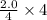
= 2.0 mol
Therefore, we can conclude that the maximum amount of C that can be produced is 2.0 mol.