Answer:
The option (e) None of the choice are correct.
Step-by-step explanation:
To find
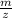 ratio in mass spectrometer,
ratio in mass spectrometer,
We start calculating from the first option
(a)
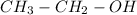
We know mass of carbon
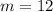 and atomic number of carbon
and atomic number of carbon
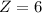
After calculating we come up with,
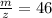
Therefore option (a) is incorrect.
(b)
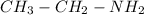
After calculating we come up with,
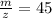
Therefore option (b) is incorrect.
(c)
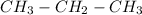
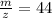
Therefore option (c) is incorrect.
(d)
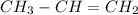
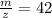
Therefore option (d) is incorrect.
Therefore, the option (e) is correct for this problem