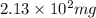Answer: The theoretical yield of 4-nitrochalcone is,
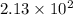
Explanation : Given,
Volume of acetophenone = 135 microliters = 135 × 10⁻⁶ L = 0.135 mL
conversion used : (1 microliter = 10⁻⁶ L) and (1 L = 1000 mL)
Density of acetophenone = 1.03 g/mL
Mass of acetophenone = Density × Volume = 1.03 g/mL × 0.135 mL = 0.139 g
Mass of 4-nitrobenzaldehyde = 127 mg = 0.127 g
Conversion used : (1 mg = 0.001 g)
First we have to calculate the moles of acetophenone and 4-nitrobenzaldehyde
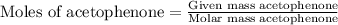
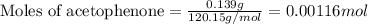
and,
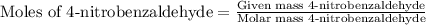
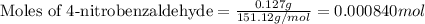
Now we have to calculate the limiting and excess reagent.
The balanced chemical equation is:
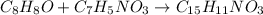
From the balanced reaction we conclude that
As, 1 mole of 4-nitrobenzaldehyde react with 1 mole of acetophenone
So, 0.000840 mole of 4-nitrobenzaldehyde react with 0.000840 mole of acetophenone
From this we conclude that, acetophenone is an excess reagent because the given moles are greater than the required moles and 4-nitrobenzaldehyde is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of 4-nitrochalcone
From the reaction, we conclude that
As, 1 mole of 4-nitrobenzaldehyde react to give 1 mole of 4-nitrochalcone
So, 0.000840 mole of 4-nitrobenzaldehyde react to give 0.000840 mole of 4-nitrochalcone
Now we have to calculate the mass of 4-nitrochalcone
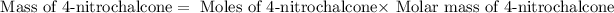
Molar mass of 4-nitrochalcone = 253.25 g/mole
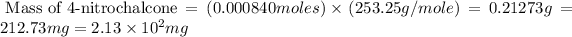
(1 g = 1000 g)
Therefore, the theoretical yield of 4-nitrochalcone is,