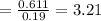Complete Question
The complete question is shown on the first and second uploaded image
Answer:
A
The percentage of water of hydration is
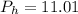 %
%
Mass of
 in 100mg is 10.60mg
in 100mg is 10.60mg
Moles of
 in 100mg is
in 100mg is
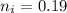
mol / mol Fe (3 sig figs) is
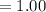
mol / mol Fe (whole number) is = 1
B
Mass of
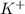 in 100mg is 27.70mg
in 100mg is 27.70mg
Moles of
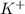 in 100mg is
in 100mg is
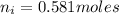
mol of K / mol of Fe (3 sig figs) is
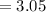
mol of K / mol of Fe (whole number) is
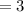
C
Mass of
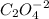 in 100mg is 55.69 mg
in 100mg is 55.69 mg
Moles of
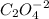 in 100mg is
in 100mg is
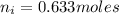
mol of
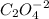 / mol of Fe (3 sig figs) is
/ mol of Fe (3 sig figs) is
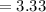
mol of
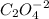 / mol of Fe (whole number) is
/ mol of Fe (whole number) is
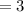
D
Mass of water in 100mg is 11.01 mg
Moles of water in 100mg is
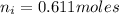
mol of water / mol of Fe (3 sig figs) is
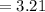
mol of water / mol of Fe (whole number) is
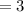
Step-by-step explanation:
The percentage of water of hydration is mathematically represented as
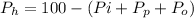
Now substituting 10.60% for
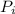 (percentage of iron ) , 22.70% for
(percentage of iron ) , 22.70% for
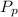 (Percentage of potassium) , 55.69% for
(Percentage of potassium) , 55.69% for
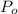 (percentage of Oxlate)
(percentage of Oxlate)
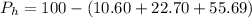
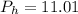 %
%
For IRON
Since the percentage of
 is 10.60% then in a 100 mg of the sample the amount of
is 10.60% then in a 100 mg of the sample the amount of
 would be 10.60 mg
would be 10.60 mg
Now the no of moles is mathematically denoted as
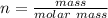
The molar mass of
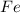 is 55.485 g/mol
is 55.485 g/mol
So the number of moles of
 in 100mg of he sample is
in 100mg of he sample is
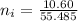
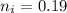
mol / mol Fe (3 sig figs) is
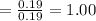
FOR POTASSIUM
Since the percentage of
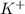 is 22.70% then in a 100mg of the sample the amount of
is 22.70% then in a 100mg of the sample the amount of
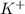 would be 22.70mg
would be 22.70mg
The molar mass of
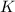 is 39.1 g/mol
is 39.1 g/mol
So the number of moles of
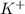 in 100mg of he sample is
in 100mg of he sample is
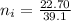
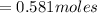
mol of K / mol of Fe (3 sig figs) is
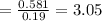
FOR OXILATE
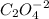
Since the percentage of
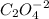 is 55.69% then in a 100mg of the sample the amount of
is 55.69% then in a 100mg of the sample the amount of
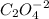 would be 55.69 mg
would be 55.69 mg
The molar mass of
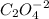 is 88.02 g/mol
is 88.02 g/mol
So the number of moles of
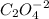 in 100mg of he sample is
in 100mg of he sample is
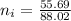
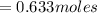
mol of
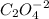 / mol of Fe (3 sig figs) is
/ mol of Fe (3 sig figs) is
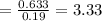
FOR WATER OF HYDRATION
Since the percentage of water is 11.01% then in a 100mg of the sample the amount of water would be 11.0 mg
The molar mass of water is 18.0 g/mol
So the number of moles of water in 100mg of he sample is
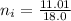
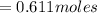
mol of water / mol of Fe (3 sig figs) is