Answer:
The volume of carbon dioxide gas generated 468 mL.
Step-by-step explanation:
The percent by mass of bicarbonate in a certain Alka-Seltzer = 32.5%
Mass of tablet = 3.45 g
Mass of bicarbonate =
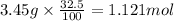
Moles of bicarbonate ion =
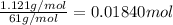
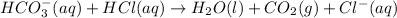
According to reaction, 1 mole of bicarbonate ion gives with 1 mole of carbon dioxide gas , then 0.01840 mole of bicarbonate ion will give:
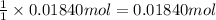 of carbon dioxide gas
of carbon dioxide gas
Moles of carbon dioxide gas n = 0.01840 mol
Pressure of the carbon dioxide gas = P = 1.00 atm
Temperature of the carbon dioxide gas = T = 37°C = 37+273 K=310 K
Volume of the carbon dioxide gas = V
 (ideal gas equation)
(ideal gas equation)
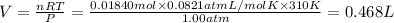
1 L = 1000 mL
0.468 L =0.468 × 1000 mL = 468 mL
The volume of carbon dioxide gas generated 468 mL.