Answer : The pH of buffer is, 5.17
Explanation : Given,
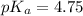
Concentration of acetic acid = 1.00 M
Concentration of sodium acetate = 1.00 M
Volume of solution = 1.00 L
As,
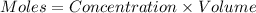
So,
Moles of acetic acid = 1.00 mol
Moles of sodium acetate = 1.00 mol
Moles of NaOH added = 0.450 mol
The balanced chemical equilibrium reaction is:
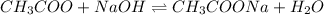
Initial mole 1 0.450 1
At eqm. (1-0.450) 0 (1+0.450)
= 0.55 =1.450
Now we have to calculate the pH of buffer.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2021/formulas/biology/college/z944fnahhldpjolfrvealc6q9baj5h69q3.png)
![pH=pK_a+\log ([CH_3COONa])/([CH_3COOH])](https://img.qammunity.org/2021/formulas/biology/college/9z631uckelzflaezrc143zx3jy64v38e5f.png)
Now put all the given values in this expression, we get:
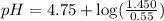
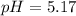
Therefore, the pH of buffer is, 5.17