Answer:
39.446L
Step-by-step explanation:
since helium is ideal gas, we can use PV = nRT
P = pressure
V = volume
n = moles
R = gas constant
T = temperature in Kelvin
we are solving for V
V =
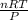
C to K temp transfer: K = C + 273, so K = 290 since C = 17
our gas constant is 0.08206 atm L/mol K, this gas constant r will change depending on what unit of pressure you are using (mmHg, atm, etc).
plug and chug
V =
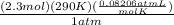
canceling out units
V =
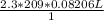 = 39.446
= 39.446