Answer:
Correct option: C
Step-by-step explanation:
As given in the question that distance between two ions are same in all cases hence r is same for all.
potential energy:
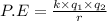
therefore potential energy depend on the two charge muliplication
so higher the charge multiplication higer will be the potential energy.
magnitude of charge multiplication follow as:
a. 1
b. 2
c. 6
d. 4
e. 2
in option C it is higher
so correct option is C