Answer:
The balanced chemical equation is given as:
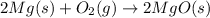
Step-by-step explanation:
When magnesium metal burns in presence of oxygen it gives white color powdered compound called magnesium oxide.
The balanced chemical equation is given as:
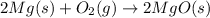
According to reaction, 2 moles of magnesium metal when reacts with 1 mole of oxygen gas it gives 2 moles of solid magnesium oxide.