For the following reaction, 76.0 grams of barium chloride are allowed to react with 67.0 grams of potassium sulfate.
The reaction consumes _____ moles of barium chloride. The reaction produces _____ moles of barium sulfate and _____ moles of potassium chloride.
Answer: a) The reaction consumes 0.365 moles of barium chloride.
b) The reaction produces 0.365 moles of barium sulfate and 0.730 moles of potassium chloride.
Step-by-step explanation:
To calculate the moles :
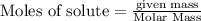
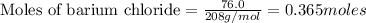
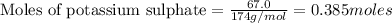
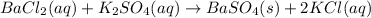
According to stoichiometry :
1 mole of
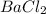 require 1 mole of
require 1 mole of
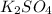
Thus 0.365 moles of
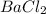 will require=
will require=
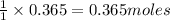 of
of
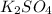
Thus
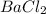 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
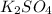 is the excess reagent.
is the excess reagent.
As 1 moles of
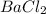 give = 1 moles of
give = 1 moles of
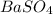
Thus 0.365 moles of
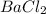 give =
give =
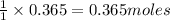 of
of
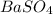
As 1 moles of
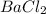 give = 2 moles of
give = 2 moles of
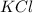
Thus 0.365 moles of
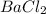 give =
give =
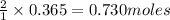 of
of
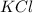
Thus the reaction consumes 0.365 moles of barium chloride. The reaction produces 0.365 moles of barium sulfate and 0.730 moles of potassium chloride.