Answer: The atomic number and mass number of the new element formed is 89 and 228 respectively.
Step-by-step explanation:
Beta decay is defined as the process in which beta particle is emitted. In this process, a neutron gets converted to a proton and an electron. The released beta particle is also known as electron.
In this process, the atomic number of the daughter nuclei gets increased by a factor of 1 but the mass number remains the same.
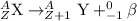
The chemical equation for the beta decay of Ra-228 follows:
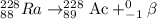
Hence, the atomic number and mass number of the new element formed is 89 and 228 respectively.