Answer:
The no. of moles present in one liter solution is 0.843
Step-by-step explanation:
Mass of ammonium chloride = 11.5 gm
Molar mass of ammonium chloride = 53.491 gm
No. of moles
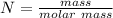
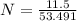
N = 0.215 moles
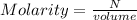
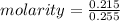
M = 0.843 M
Thus the no. of moles present in one liter solution is 0.843