Answer : The final concentration of ONGP is, 0.47 mM
Explanation :
Formula used :

where,
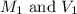 are the initial molarity and volume
are the initial molarity and volume
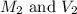 are the final molarity and volume
are the final molarity and volume
We are given:
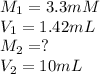
Now put all the given values in above equation, we get:
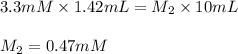
Hence, the final concentration of ONGP is, 0.47 mM