Answer:
21.28 grams solute can be added if the temperature is increased to 30.0°C.
Step-by-step explanation:
Solubility of solute at 20°C = 32.2 g/100 grams of water
Solute soluble in 1 gram of water =
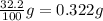
Mass of solute in soluble in 56.0 grams of water:
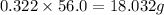
Solubility of solute at 30°C = 70.2g/100 grams of water
Solute soluble in 1 gram of water =
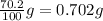
Mass of solute in soluble in 56.0 grams of water:
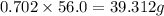
If the temperature of saturated solution of this solute using 56.0 g of water at 20.0 °C raised to 30.0°C
Mass of solute in soluble in 56.0 grams of water 20.0°C = 18.032 g
Mass of solute in soluble in 56.0 grams of water at 30.0°C = 39.312 g
Mass of of solute added If the temperature of the saturated solution increased to 30.0°C:
39.312 g - 18.032 g = 21.28 g
21.28 grams solute can be added if the temperature is increased to 30.0°C.