Answer:
Reaction is spontaneous at high temperature and nonspontaneous at low temperature
Step-by-step explanation:
Given:
Enthalpy change
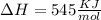
Entropy change
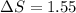
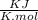
From the formula of change in free energy,
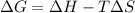
But for spontaneous process the values of quantities are given below
For spontaneous process value of
 is negative
is negative
For nonspontaneous process value of
 is positive
is positive
Here values of
 and
and
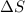 are positive, so reaction is spontaneous at
are positive, so reaction is spontaneous at
high temperature and nonspontaneous at low temperature