Answer : The amount of energy absorbed is, 81.2 kJ
Explanation :
The process involved in this problem are :
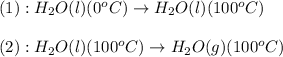
The expression used will be:
![Q=[m* c_(p,l)* (T_(final)-T_(initial))]+[m* \Delta H_(vap)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/4ne3y7nh13qhu5mkethkfk8rfkis4l78s3.png)
where,
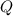 = heat required for the reaction = ?
= heat required for the reaction = ?
m = mass of liquid = 30.3 g
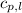 = specific heat of liquid water =
= specific heat of liquid water =
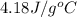
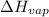 = enthalpy change for vaporization =
= enthalpy change for vaporization =
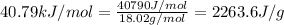
Now put all the given values in the above expression, we get:
![Q=[30.3g* 4.18J/g^oC* (100-0)^oC]+[30.3g* 2263.6J/g]](https://img.qammunity.org/2021/formulas/chemistry/high-school/9nilv01cmjusovuyhwhgs90e9jkel595u5.png)
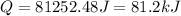
Therefore, the amount of energy absorbed is, 81.2 kJ