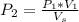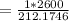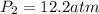Complete Question
A marine biologist is preparing a deep-sea submersible for a dive. The sub stores breathing air under high pressure in a spherical air tank that measures 74.0 wide. The biologist estimates she will need 2600 L of air for the dive. Calculate the pressure to which this volume of air must be compressed in order to fit into the air tank. Write your answer in atmospheres. Round your answer to significant digits.
Answer:
The pressure required is
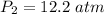
Step-by-step explanation:
Generally the volume of a sphere is mathematically denoted as
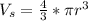
Substituting
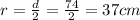
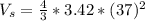
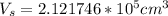
Converting to Liters
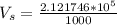
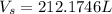
Assume that the pressure at which the air is given to the diver is 1 atm when the air was occupying a volume of 2600L
So
From Charles law
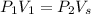
Substituting
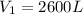 ,
,
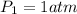 ,
,
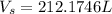 , and making
, and making
 the subject we have
the subject we have