Answer:
1.06M
Step-by-step explanation:
Given parameters:
Mass of HBr = 50g
Volume of water = 600mL
unknown:
Molarity of the solution = ?
Solution:
The molarity of a substance is the number of moles of solute in a volume of solution.
Mathematically;
Molarity =
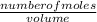
Since the number of moles is unknown, let us solve for it;
Number of moles =

Molar mass of HBr = 1 + 80 = 81g/mol
Number of moles =
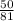 = 0.64mole
= 0.64mole
Convert the volume to liters or dm³; 600mL = 0.6L or 0.6 dm³
Input the parameters and solve;
Molarity =
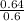 = 1.06M
= 1.06M