Answer:
0.11mole
Step-by-step explanation:
Let us assume that the condition is at standard temperature and pressure(STP);
Given parameters:
Volume of water = 2.45L
Unknown:
Number of moles found in this volume of water = ?
Solution;
At STP;
Number of moles =
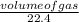
Input the parameters and solve;
Number of moles of water =
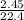 = 0.11mole
= 0.11mole
The number of moles of water found is 0.11mole