The size of volumetric flask is 0.5L = 500mL
Step-by-step explanation:
In order to estimate the concentration of a solution in molarity, then the total number of moles of the solute is divided by the total volume of the solution.
Molarity of a solution is given by;
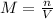
Where;
n= number of moles of solute
V= volume of solution = size of volumetric flask
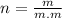
Where;
m= mass of solute=43.85g
m.m= molar mass of solute=58.5g
Note that solute is NaCl(s)
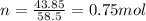
We know that the molarity is 1.50M
To find the size of the volumetric flask, we make V in M=n/V subject of formula
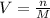
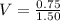
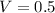
Therefore, the size of volumetric flask is 0.5L = 500mL.