The rate of effusion of gas B is 728.32 m/s
Step-by-step explanation:
Given:
Mass of A, m₁ = 46 g/mol
Rate of effusion of A, R₁ = 515 m/s
Mass of B, m₂ = 92 g/mol
Rate of effusion of B, R₂ = ?
We know:
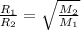
Substituting the value we get:
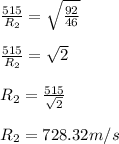
Therefore, the rate of effusion of gas B is 728.32 m/s