Answer:
1.4
Step-by-step explanation:
Mass of pure nitric acid = 751mg
Volume of solution = 290mL
Unknown:
pH of the solution = ?
Solution:
To solve this problem, we need the concentration of the acid in the aqueous form.
This is given by molarity;
Molarity =
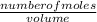
Since the number of moles of nitric acid is unknown, we can easily solve for it.
Number of moles of nitric acid =

molar mass of HNO₃ = 1 + 14 + 3(16) = 63g/mol
mass of nitric acid = 751mg = 0.751g
Number of moles =
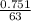 = 0.012mole
= 0.012mole
Volume of solution = 290mL = 0.29dm³
Now molarity of the solution =
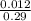 = 0.041moldm⁻³
= 0.041moldm⁻³
Since:
pH = -log [H₃O⁺]
HNO₃ + H₂O → H₃O⁺ + NO₃⁻
1moldm⁻³ 1moldm⁻³ 1moldm⁻³
0.041moldm⁻³ 0.041moldm⁻³ 0.041moldm⁻³
pH = -log[0.041] = 1.4