Question options:
a) 2.05
b) 0.963
c) 0.955
d) 1.00
Answer:
b) 0.963
Step-by-step explanation:
H2SO4→ HSO4- + H3O+
HSO4- + H2O ⇌ SO42- + H3O+
Construct ICE table:
HSO4- (aq) + H2O ⇌ SO42- (aq) + H3O+ (aq)
I 0.1 solid & 0 0.1
C -x liquid + x + x
E 0.1 - x are ignored x 0.1 + x
Calculate x
Ka = products/reactants
=
![([SO42-] [H3O+])/([HSO4-])](https://img.qammunity.org/2021/formulas/chemistry/college/m0nla76vq2nsvb70v5ji33hr79s7ld4fbw.png)
0.011 =
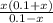
0.011 x (0.1 -x) = o.1x + x^2
0.0011 - 0.011 x - o.1x - x^2 = 0
0.0011 - 0.011 x - x^2 = 0
Use formula to solve for quadratic equation
x =
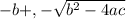 / 2a
/ 2a
a = -1, b = -0.111, c = 0.001
Solve for x
x =
![\sqrt[-(-o.111)]{(-0.111)^2 - 4(-1) (0.0011) }](https://img.qammunity.org/2021/formulas/chemistry/college/jx3actfgekdi6gswt9k6993pzohdkemfv4.png) / 2(-1)
/ 2(-1)
x = 0.111 +,-
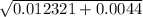 / -2
/ -2
x = 0.111 +,-
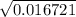 / -2
/ -2
x =
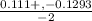
x =
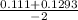 , x =
, x =
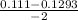
x =
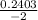 , x =
, x =
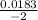
x = - 0.12015 , x = 0.00915
x cannot be negative, so
x = 0.00915 M
Calculate [H3O+]
[H3O+] = 0.1 M + x
[H3O+] = 0.1 M + 0.00915 M
[H3O+] = 0.10915 M
Clculate pH
pH = - log [ H3O+]
pH = - log [ 0.10915]
pH = 0.963