2 . The ratio of 1:2 in the mass of second element, oxygen in CO and CO2 verifies the law of proportion.
3.
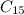
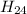
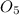 is the molecular formula of the new malaria drug given.
is the molecular formula of the new malaria drug given.
Step-by-step explanation:
Balanced chemical reaction:
C + O ⇒ C0
C + O2 ⇒ CO2
law of multiple formulation states that when two elements combine to form a compound the ratio of the mass of elements is a whole number.
Carbon reacts with oxygen to produce:
Mass of carbon mass of oxygen ratio of O in CO2 to O in CO
CO = 12 16 3:4
CO2 = 12 32 3:8
CO 6 gm 8 3:4
CO2 6 16 3:8
The ratio of the mass of the second element i.e oxygen is CO:CO2 is 1:2, hence it shows law of multiple proportions.
3. given,
(C5H8)nO5 = emperical formula
relative molecular mass = 284
molecular formula= ?
The value of n will be calculated as:
(12x5)+ (1x8)n + (16x5) = 284
(60 +8)n + 80 = 284
68n + 80 = 284
68n = 284 - 80
68n = 204
n = 3
Multiplying the emperical formula by n
(C5H8)3O5
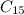
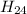
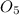 is the molecular formula of the drug.
is the molecular formula of the drug.