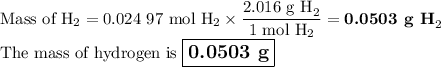Answer:
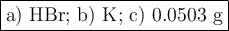
Step-by-step explanation:
The limiting reactant is the reactant that gives the smaller amount of product.
Assemble all the data in one place, with molar masses above the formulas and masses below them.
M_r: 39.10 80.41 2.016
2K + 2HBr ⟶ 2KBr + H₂
m/g: 5.5 4.04
a) Limiting reactant
(i) Calculate the moles of each reactant
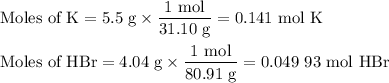
(ii) Calculate the moles of H₂ we can obtain from each reactant.
From K:
The molar ratio of H₂:K is 1:2.
From HBr:
The molar ratio of H₂:HBr is 3:2.
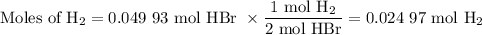
(iii) Identify the limiting reactant
HBr is the limiting reactant because it gives the smaller amount of NH₃.
b) Excess reactant
The excess reactant is K.
c) Mass of H₂