Answer:
0.426 L
Step-by-step explanation:
Boyles law is expressed as p1v1=p2v2 where
P1 is first pressure, v1 is first volume
P2 is second pressure, v2 is second volume.
Given information
P1=96 kPa, v1=0.45 l
P2=101.3 kpa
Unknown is v2
Making v2 the subject from Boyle's law
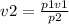
Substituting the given values then
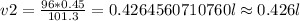
Therefore, the volume is approximately 0.426 L