Answer:
52.80 % is the percent yield of the reaction.
Step-by-step explanation:
Mass of nitrogen gas = 38.0 g
Moles of nitrogen =
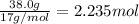
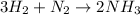
According to reaction, 1 moles of nitrogen gas gives 2 moles of ammonia, then 2.235 moles of nitrogen will give:
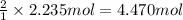 ammonia
ammonia
Mass of 4.470 moles of ammonia
= 4.470 mol × 17 g/mol = 75.99 g
Theoretical yield of ammonia = 217.8 g
Experimental yield of ammonia = 40.12 g
The percentage yield of reaction:
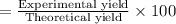
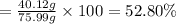
52.80 % is the percent yield of the reaction.