The molarity is 3.50 M/L.
Step-by-step explanation:
Molarity is found to know the amount of the solute ions present in the given volume of solution. So we determine it using the ratio of the moles of solute to the volume of the solution.
As here the weight of the solute which is hydrochloric acid is given as 17g, so we need to find the moles of it. This can be done by dividing the given amount of HCl with the molecular weight of HCl.
Molecular weight of HCl = 1.01 + 35.45 = 36.46 g/mol
So the number of moles of HCl present here will be
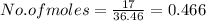
So, 0.466 moles of HCl is present in the solution.
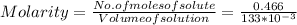
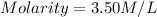
Thus, the molarity is 3.50 M/L.