The concentration of the HCl solution is 0.047 M.
Step-by-step explanation:
Data given about acid and base:
volume of acid Vacid = 46.9 ml
molarity of acid =?
volume of the base (NaOH) = 16.4 ml
molarity of the base = 0.135 M
To know the concentration of the acid in this reaction, the formula used in titration is used. It is
Macid X Vacid = Mbase X Vbase
the formula is rewritten as:
Macid =
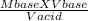
putting the values in the equation:
Macid =
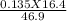
= 0.047 M
the concentration of the acid i.e HCl in the solution is of 0.047 M.