Answer:
0.178 moles of oxygen gas molecules were collected.
Step-by-step explanation:
Pressure at which oxygen gas collected = p= 1.00 atm
Vapor pressure of the water
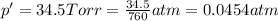
Pressure of the oxygen gas = P
p = p' + P
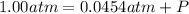
P = 1.00 atm - 0.0454 atm = 0.9546 atm
Volume of the oxygen gas = V = 4.56 L
Moles of oxygen gas = n
Temperature at which gas collected = T =
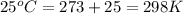
 (ideal gas)
(ideal gas)
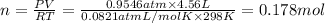
0.178 moles of oxygen gas molecules were collected.