92.8 % is the percent yield when 2.87 g of aluminum are reacted with excess copper (II) sulfate and 9.2 g of copper are produced.
Step-by-step explanation:
Mass of aluminum = 2.87 grams
mass of copper produced = 9.2 grams ( actual yield)
the balanced chemical reaction is given as:
2 Al + 3 CuSO4 ⇒ ( AL2(SO4)3 +3 Cu.
from the reaction it is found that
2 moles of aluminum reacted to give 3 moles of copper
so converting them in to mass.
number of moles of copper in 2.8 grams =
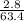
number of moles = 0.104 moles of copper
atomic mass of aluminum = 26.98 grams/mole
atomic mass of copper = 63.54 grams/mole
From the given data
2.87 grams Al gives 9.2 grams of Cu.
2 moles of aluminum reacted to give 3 moles of copper
0.104 moles of aluminum will give
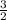 =
=
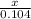
2x = 0.132
x = 0.156 moles of Cu will be formed.
Hence theoretical yield should be = 0.156 x 63.54
= 9.91 grams
Percent yield =
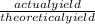 x 100
x 100
=
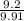 x 100
x 100
percent yield = 92.8 % is the yield percent.