Answer:
94.8% is the percentage yield of this reaction.
Step-by-step explanation:
Mass of aluminium = 3.8 g
Moles of aluminium =
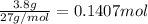
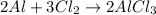
According to reaction, 2 moles of aluminum gives 2 moles of aluminium chloride, then 0.1407 moles of aluminium will give:
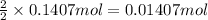 aluminium chloride
aluminium chloride
Mass of 0.1407 moles of aluminum chloride:
= 0.1407 mol × 133.5 g/mol = 18.78 g
Theoretical yield of aluminum chloride = 18.78 g
Experimental yield of aluminum chloride = 17.8 g
The percentage yield of reaction:
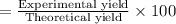
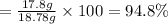
94.8% is the percentage yield of this reaction.